Toluene is an important chemical used in industries such as manufacturing and scientific research. It is of interest to experts and learners alike because of its unique chemical properties and multiple uses. One critical trait pertaining to toluene’s behavior and usability is its melting point. This article investigates the captivating realm of toluene, analyzing its specific features alongside melting point and discussing other broader notions regarding chemistry and industry. We will simplify the complex aspects of toluene while illustrating its significance, intending to appeal to scholars, professional chemists, and even laypeople interested in the science surrounding mundane substances.
What is the chemical structure of toluene?

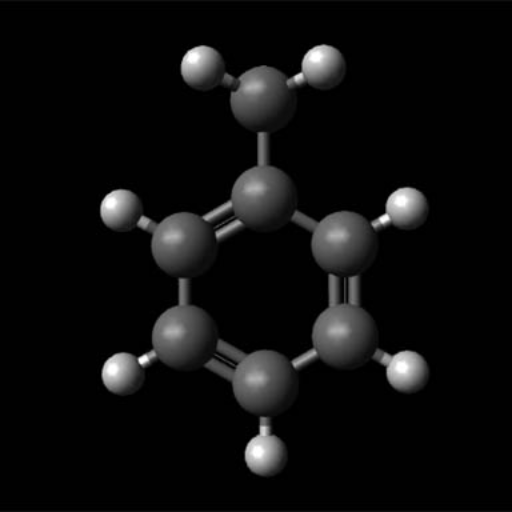
Toluene’s chemical structure is relatively simple in that it has a methyl group (CH3) attached to a benzene ring, which is a hexagonal ring of six carbon atoms with alternating double bonds. Its molecular formula is C7H8, which classifies it as an aromatic hydrocarbon.
Studying Toluene as Methylbenzene
The name methylbenzene comes from the fact that toluene has a benzene ring and methyl group. The structure provides high stability and chemical adaptability. As an aromatic hydrocarbon, toluene has a benzene ring which follows Huckel’s Rule for aromaticity. The presence of aromaticity plays an important factor in determining the reactivity of toluene and its functions in a chemical reaction.
Toluene appears as a colorless and clear liquid. It also has a characteristic odor similar to that of paint thinner. The substance melts at -95 °C (-139 °F) and boils at approximately 110.6 °C (231.1 °F). When the temperature is 20 °C, toluene has a density of 0.867 g/cm³, which is less than the density of water. The properties described above makes toluene a very good solvent used both in labs and in industry.
Toluene is used extensively in the formulation of paints, thinners, adhesives, and as a cleansing agent. Further, its use in gasoline as an octane booster adds to the efficiency of the engine by resisting knocking. In the chemical industry, toluene is a critical feedstock as it is used to produce benzene, toluene diisocyanate which is used in the production of polyurethanes, and other petrochemical derivatives.
As useful as toluene is, it does require special precautions when handling due to its negative effects on health and the environment. Chronic exposure to toluene vapors can result in effects such as dizziness, headaches, and more extreme neurological damage due to the centr nervous system being affected. Furthermore, toluene can pose environmental risks such as damaging aquatic ecosystems. Thus, workplaces that handle toluene need to follow compliance guidelines such as exposures limits set by OSHA and NIOSH.
Comparison with Benzene: Structural Differences
Toluene differs from benzene in structure due to the presence of a methyl group (-CH3) attached to the benzene ring in toluene, whereas benzene consists of just a six-carbon ring with alternating double bonds.
| Key Point | Benzene | Toluene |
|---|---|---|
| Molecular formula | C6H6 | C7H8 |
| Functional group | None | Methyl (-CH3) |
| Structure | Simple ring | Methyl ring |
| Boiling point | ~80°C | ~111°C |
| Polarity | Nonpolar | Slightly polar |
| Reactivity | Lower | Higher |
| Environmental impact | Toxic | Less toxic |
The Influence of the Methyl Group on Toluene
The methyl group (-CH3) attached to toluene changes its properties relative to benzene, which might be due to its electron donating inductive effect. This substituent makes toluene more reactive to electrophilic substitution reactions like nitration, sulfonation, and halogenation. Enhanced reactivity to these types of reactions is one of the key differences between toluene and benzene, which tends to be less reactive under similar conditions.
With regards to physical properties, the presence of the methyl group also changes the boiling and melting points of toluene. Toluene’s boiling and melting points are around 110.6°C and -95°C respectively, while benzene’s boiling and melting points are 80.1°C and 5.5°C. This comparison further illustrates the difference made by the methyl group concerning molecular interactions and thermal stability.
From an industrial perspective, even though toluene has a lower solubility in water than benzene due to the methyl group which makes the molecule more hydrophobic, it is still an excellent solvent for many other organic compounds. For this reason, toluene is Widely used in industries such as paint thinners, adhesives, and chemical synthesis.
Recent research highlights that the methyl group present in toluene impacts its environmental effects along with the health-related issues. While toluene exposure is monitored due to its potential toxicity, its methyl substitution alleviates some of the risks when compared to benzene, a known carcinogen.
How does the melting point of toluene influence its use?

The melting point of toluene which is approximately -95°C (-139°F) means it can be used in low-temperature environments and applications. The low melting point of toluene enhances its usefulness as a solvent and reactant in industrial chemical processes.
Toluene’s Property as a Liquid at Room Temperature
As mentioned before, the melting point of toluene is -95°C and has a boiling point of around 111°C (232°F). This easily allows toluene to remain a liquid at room temperature. Toluene’s stability under these conditions makes it useful across numerous industries. More recent data shows that toluene is a solvent of choice in the industrial to manufacture paints, coatings, adhesives, and inks owing to its high solvency power. Furthermore, toluene’s easily accessible nature as a precursor in the synthesis of benzene, phenol and nylon has rendered it an essential compound in petrochemical production.
Further details indicate that toluene is preferred for commercial use owing to its low volatility toxicity when compared to other volatile organic compounds, alongside its chemical stability. The global demand for Toluene is on the rise, and as of 2023, the toluene market is valued at $23 billion and projected to increase to $28 billion by 2030 with a CAGR of 3%. This increasing demand signifies its importance throughout all sectors including automotive and construction.
Applications in Industrial Processes
Due to the wide variety of industrial applications, toluene has become essential in several processes. One of the most important uses is in the production of benzene, which is essential in creating plastics as well as resins and synthetic fibers. Furthermore, toluene is a solvent in the production of paint, coatings, adhesives, and rubber because it can dissolve many chemical compounds.
Recent studies show that toluene is also widely used in the production of polyurethane foam, which is key insulation material as well as for furniture and automotive seating. The global polyurethane foam market is estimated to grow at a 6.7% CAGR between 2023 and 2030, thus indirectly increasing demand for toluene.
Another rising use is as a fuel additive. The global gasoline market is expected to grow to $450 billion by 2028 which also emphasizes toluene’s sustained importance in the domain. Moreover, toluene’s high-octane rating makes it an effective gasoline anti-knocking agent which improves engine efficiency. In addition, toluene is a primary raw material for producing toluene diisocyanate (TDI) which is used in the manufacturing of flexible foams and coatings.
These cases showcase toluene’s adaptability which evolves alongside new industrial innovations and trends in sustainability, reinforcing the continued significant economic and industrial reason to strengthen the usage of toluene.
Why melting point matters in organic chemistry
The melting point is one of the fundamental physical properties a compound possesses and serves as an identifier in organic chemistry. In addition, it helps categorize the purity and stability of the substances. Most frequently, a sharp range of 1-2°C signifies the presence of a singular compound. The addition of impurities can greatly lower or widen the aforementioned range. Benzoic acid is a common organic compound which is said to have a melting point of 122.4°C. Deviation from this is an indication of some form of contamination or adulteration.
Additionally, the melting point plays a significant role in determining the intermolecular forces operating in a compound. Compounds that are
carboxylic acids or alcohols, which contain strong hydrogen bonding, tend to have higher melting points than those with weaker dispersion forces. In the
pharmaceutical industry, precise industrial melting point measurements are crucial due to the stringent requirements for consistency and stability
within compounds. As an example, some drugs need to be kept at specific temperatures during storage, and thus a substance’s melting point may
directly influence its formulation, shelf life, or even stability.
Other recent studies focus on the importance of melting point from the sustainability perspective. For instance, scientists are working on
biodegradable polymers with customized melting points to enhance environmental degradation. These examples illustrate the versatile application of
melting point analysis, the one that continues to stimulate inventive work in numerous disciplines.
What are the chemical properties of toluene?

Toluene, or toluol, is an aromatic and aliphatic hydrocarbon, colorless in appearance, with the molecular formula C₆H₅CH₃. Its boiling and melting points are approximately 110.6°C and -95°C, respectively. Due to the nature of its chemical structure, toluene is very soluble in organic solvents, while not so in water. It is also flammable, sweet, and pungent. Chemically, toluene has moderate methyl substitution reactivity. Because of these properties, toluene is very common in the manufacturing, pharmaceutical and other industrial sectors.
Toluene as an aromatic hydrocarbon
Due to the presence of toluene’s benzene ring, which is characteristic of aromatic compounds, it is classified as an aromatic hydrocarbon. The methyl group bonded to the benzene ring does change its chemical properties profoundly, but still retains the aromaticity required to be classified as toluene.
Solvent Properties and Industrial Uses
As a solvent, toluene has some effectiveness because it dissolves many organic compounds. Industries utilize it for the production of paints, coating, adhesives, and inks where its solvent properties guarantee uniformity, consistency, and quality during application. Moreover, toluene is utilized in the production of important chemicals like benzene and xylene through industrial processes such as catalytic reforming.
The pharmaceutical industry utilizes toluene as a solvent for reactions due to its ability to dissolve certain chemicals and stabilize reactive primers. Industries depend on a toluene’s physical properties like boiling point of 110.6°C (231 °F), and density of 0.87 g/cm³ for favorable processing conditions.
As industrial solvents and for petrochemical production, toluene consumption is forecasted to exceed 25 million metric tons by 2025. Its extensive use in processes of aromatic hydrocarbons illustrates its importance in contemporary industrial tasks and reinforces its position in international value chains.
Analyzing Diethyl Ether and Toluene
Both toluene and diethyl ether are important solvents in an array of industrial and chemical practices. Their applications differ greatly. In toluene’s case, is widely utilized for producing benzene and other aromatic compounds. Solvents like paint thinners, adhesives and chemical extraction apparatuses also employ toluene. Moreover, toluene’s boiling point stands at 110.6°C (231°F) and its density is 0.87 g/cm³. In comparison, diethyl ether has a lower boiling point of 34.6°C (94.3°F) and its density is at 0.713 g/cm³ which makes it more flammable and volatile.
Recent data shows that toluene is continuously gaining demand all over the globe, especially in the petrochemical industry which relies heavily on the compound because it is needed to produce toluene diisocyanate (TDI) along with several other derivatives used for manufacturing foams, coatings, and elastomers. This in turn drives the need to consume over 25 million metric tons of toluene by the year 2025. Diethyl ether’s specific medical and scientific applications put it in a tiny market segment and aside from the laboratory usage and manufacturing anesthetics, ethers don’t have much mainstream industrial appeal. In spite of this, ethers are still used due to diethyl ether having a low boiling point and being able to effectively serve as a solvent.
Differences in solvent procedures arise from their environmental impacts as well. Toluene is categorized as a volatile organic compound (VOC) and poses concerns for both air quality and workplace exposure. On the other hand, ethers are highly flammable and, moreover, form peroxides that are exceedingly dangerous. Such factors accentuate the understanding of industrial and research intended roles, applications, and safety measures towards handling industrial solvents.
How is toluene used in the chemical industry?

Due to its ability to dissolve various chemical compounds, toluene is widely used in the chemical industry as a solvent for paints, coatings, and even adhesives and inks. Its utility as a feedstock in the synthesis of other chemicals, such as benzene and xylene, makes it a crucial component in other chemical processes. It also aids in the manufacturing of polymers, plastics, and synthetic fibers. Furthermore, toluene enhances the octane levels in gasoline. All of these applications illustrate its wide-ranging utility in different industrial processes.
Toluene: From Paint to Adhesive and Everything in Between
To toluene’s unique chemical properties, it is essential in several industries. In adhesives, toluene is performed as a solvent, creating the right consistency for refined viscosity, as well as for easy application. This is critical for the production of pressure-sensitive adhesives and formulations based on synthetic rubbers. In the paint and coatings industry, toluene serves as a fast evaporating solvent that enables the smoothing of finishes, reduction of drying times and efficient dispersion of pigments.
The most recent data underscores the significance of toluene on a global scale. As per toluene market research, its value stood at $23 billion in 2022 with a projected compound annual growth rate (CAGR) of 4.5% from 2023 to 2030, owing to heightened demand in automotive paint CHP’s and a surge in worldwide construction projects. Furthermore, the importance of toluene extends to its derivatives such as benzene and toluene diisocyanate (TDI) which are crucial in the production of polyurethanes used in products like insulation, foam seating, and other domestic goods.
Another critical use for toluene is as an octane booster which highlights further versatility. Addition of toluene aids in improving engine performance and decreasing the likelihood of knocking, thus providing more dependable and sustainable oil and gas resources. Thus far, toluene remains to be a crucial resource for industrial advancements and innovations, directly owing to the consistent demand from manufacturers and industries.
The Manufacturing of Rubber and Printing Inks
The use of toluene is crucial in the rubber and printing ink industry because of its strong Solvent properties. In the rubber sector, toluene is often used in the vulcanization of rubber, as it dissolves synthetic polymers, to ensure proper mixing and curing of the rubber materials. This chemical process ensures that high quality and durable rubber goods are achieved which are crucial in automotive, construction, and consumer goods industries.
As for printing inks, toluene is a major part of ensuring smooth and consistent application of the ink. It also helps in effective dispersal of dyes and pigments which in turn helps the performance of inks in high speed printing processes because toluene is a high solvating power solvent. In 2023, market research indicates that the global printing ink market is expected to increase by 4.1% CAGR, from which the market for industrial solvensts is projected to grow significantly because toluene solvent has contrubuted and will continue to contribute a great deal towardm this growth. The increase is coming from the packaging, publication and digital printing sectors that prefer toluene based inks because of their consistency and better resistance to smudging.
In this case, toluene serves as an archetype for chemicals that greatly enhance modern industrial processes, as it stimulates the innovation of materials and products.
Toluene as a Gasoline and Fuel Additive
Toluene is vitally important in the production of gasoline and other fuel additives for its high octane rating as it improves performance and engine efficiency. Toluene, an aromatic hydrocarbon, is commonly applied in the petroleum industry to increase the quality of fuel. Hefty modern gasolines usually contain about 5-15% of toluene which enhances the octane rating. This reduces knocking in the engine and contributes to the overall vehicle performance in a smoother operation.
As the International Energy Agency (IEA) reported, toluene consumption is expected to steadily increase worldwide due to the rising consumption of fuel additives in emerging economies. Moreover, studies by industry researchers show that the gasoline sector in toluene is expected to reach an estimated value of 30 billion dollars by the year 2027, signifying a compound annual growth rate (CAGR) of approximately 3.5% starting from 2023.
In addition to refining fuel to a desired level, toluene helps reduce emissions by allowing for cleaner burning processes. This indicates that toluene may be critical to sustainable innovation in energy and transportation as toluene’s role in innovation will surely change in response to ongoing innovation towards lower emission fuels.
Where can I find reference data on toluene?
- The United States Environmental Protection Agency (EPA): Offers access to detailed documents concerning toluene’s environmental and health effects. Visit www.epa.gov.
- The National Center for Biotechnology Information (NCBI): Specializes in the chemical properties and applications of toluene. Search through Pubchem at www.ncbi.nlm.nih.gov.
- The Occupational Safety and Health Administration (OSHA): Regulates compliance and safety instruction concerning the use of toluene. Visit www.osha.gov for more information.
- Industry-specific reports and market analyses: Statista and other research firms have been known to publish toluene market analyses.
Scientific and U.S. Regulatory Reference Sources
- Environmental Protection Agency (EPA): Provides toluene environmental impact assessment, regulation documents and guidelines for its use on www.epa.gov.
- The National Institute for Occupational Safety and Health has published www.cdc.gov/niosh extensive documents regarding toluene’s health hazard, static safety guidelines as well as permissible exposure levels.
- Chemical Safety and Hazard Investigation Board (CSB): Publishes investigative reports on various toluene industry accidents and recommendations on safety measures to avert their recurrence. Their website is www.csb.gov for further details.
Accessing chemical databases and journals
You may access the chemical databases and journals by using the PubChem, SciFinder, or PubMed for in-depth information about the chemicals and their safety measures. Also, most universities have subscriptions to particular chemistry and safety journals and the American Chemical Society (ACS) maintains a large collection of research databases.
Reference sources
- Title: Catalytic Cracking of Toluene as a Tar Model Compound Using Sewage-Sludge-Derived Char
- Authors: P. Lu et al.
- Journal: Energy & Fuels
- Publication Date: 2016-09-09
- Key Findings:
- The study investigates the catalytic cracking of toluene, a model tar compound, using char derived from sewage sludge.
- It examines the effects of various syngas components on the conversion ratio of toluene and the distribution of cracking products.
- The results indicate that the highest conversion ratio of toluene was achieved at 950 °C, with significant improvements in cracking efficiency when CO2 and steam were present.
- Methodology:
- The experimental setup involved varying reaction atmospheres and temperatures to assess the catalytic performance of the sewage sludge char.
- The study utilized analytical techniques to measure conversion ratios and product distributions.
- Title: Prediction of Solubility of Racemic (R/S) (±)-Ibuprofen in n-Heptane, Toluene, Benzene and Ethanol
- Authors: H. Bagheri, N. Hatami
- Publication Year: 2017
- Key Findings:
- This research focuses on predicting the solubility of racemic ibuprofen in various solvents, including toluene.
- The study provides insights into the solubility behavior of ibuprofen in relation to its melting point and the solvent’s properties.
- Methodology:
- The authors employed experimental solubility measurements and theoretical models to predict solubility in different solvents.
- The results were analyzed to understand the interactions between ibuprofen and the solvents.
- Title: A simple technique for preparing low-melting-point samples for neutron powder diffraction
- Authors: R. Ibberson
- Journal: Journal of Applied Crystallography
- Publication Date: 1996-08-01 (not within the last 5 years but relevant)
- Key Findings:
- The paper presents a method for preparing low-melting-point samples, including toluene, for neutron powder diffraction studies.
- The technique involves freezing and hand-grinding the material to produce high-quality powder samples.
- Methodology:
- The author describes the preparation process in detail, emphasizing the importance of maintaining sample integrity for accurate diffraction results.
Frequently Asked Questions (FAQs)
Q: What is the melting point of toluene?
A: Toluene’s melting point is about -95°C (-139°F).
Q: Is toluene soluble in water?
A: Toluene does not dissolve in water and is regarded as immiscible with water. However, it can be dissolved by organic solvents such as ethanol, acetone, and carbon disulfide.
Q: What are the common uses of toluene?
A: Toluene is utilized as a solvent in the manufacture of paint, nail polish, and lacquer. It is also used in gasoline, and in the production of chemicals, for example, trinitrotoluene (TNT).
Q: Is toluene toxic?
A: Inhalation and ingestion of large quantities of toluene makes one toxic and so toluene is regarded as toxic. There is a need to control toluene exposure and ensure adequate room ventilation during the use of products containing toluene where exposure levels are monitored.
Q: Can toluene be used as a recreational inhalant?
A: Although some people misuse toluene as a recreational inhalant because of its psychoactive effects, doing so is exceptionally hazardous and can result in considerable health risks, including harm to the nervous system.
Q: Where can toluene be found as a natural resource?
A: Toluene can be found naturally in crude oil and as a byproduct in coking plants where coal undergoes dry distillation to form coke. Via industrial processes and vehicle exhaust, toluene can also be released to the environment.
Q: What type of a compound is toluene and how does it relate with mono substituted benzene derivatives?
A: Toluene, as a mono-substituted benzene derivative, incorporates a methyl group to a singular position on the benzene ring, thus altering its chemical properties and applications.
Q: Is glacial acetic acid miscible with toluene?
A: Toluene is miscible with glacial acetic acid meaning the two can combine in any proportions while still remaining homogeneous.
Q: What can be said about the oxidation of toluene?
A: Under the right conditions, particularly in the presence of a catalyst, toluene undergoes oxidation producing benzaldehyde and benzoic acid, important chemicals used in various industrial processes.
Q: What is the use of toluene in leather finishing?
A: In the leather industry, toluene serves as a solvent during the leather finishing and treatment processes, assisting in the dissolution of other materials as well as in the application and drying stages.

