Under intense and harsh environmental conditions, corrosion and rust-proofing of the materials may be found to be the most important. Titanium – also known as the “wonder metal” – is the most widely used material in many industries from aerospace to orthopaedic implantation. Titanium is a ‘Super’ metal, as many prefer to call it, but it also rusts with other metals, and if so, what is the basis of its strength as a non-rusting metal?
This piece aims to investigate the underlying reasons behind titanium’s behavior, its interactions with its surroundings, and, more specifically, how it outcompetes traditional metals under adverse conditions. Suppose you’re seeking answers on titanium and the implications in high-performance engineering, or looking into why it is a game-changer for some sectors. In that case, a dissertation provides evaluations of the alloys’ resistance to corrosion, posing the all-too-relevant inquiry: Does titanium last long, or can it rust very quickly as well?
Debunking the Titanium Rust

No, titanium does not rust. The reaction to convert metals into other compounds is a physicochemical process that happens as a result of oxygen and moisture. Titanium is under no oxidation because of the presence of Iron; it is almost free of Iron in its composition, so it simply does not rust. Instead, titanium forms a shiny tin oxide layer that is exceptionally well bonded to the metal and does not change. Once this layer is formed, it prevents the metal from further reactions. This type of titanium, which has a passive layer, is not readily reactive. The skin layer of titanium is very slow to chemical breakdown. This is why titanium is used to craft elements exposed to harsh conditions or chemicals, including saltwater erosion, but still are expected to last longer than their quaint appearance.
What is Rust?
Rust, in the realm of materials and chemistry, is a form of corrosion that primarily affects iron and its alloys, such as steel. Secondly, it occurs when iron reacts with available oxygen and absorbs moisture, yielding ferrous oxide. This reddish-brown iron oxide forms streaks on the metal’s surface, simultaneously weakening it. The metal will bow and transform its shape due to this corrosion. In some instances, the process of metal corrosion, known as rusting, accelerates. Salty and acidic environments usually cause this, as they contain electrolytes that enhance the reaction rates.
Titanium’s Unique Properties
Titanium is a unique metal due to its exceptional combination of properties, making it valuable in numerous industries. The following are five significant titanium properties:
- High Strength-to-Weight Ratio: Titanium is as strong as steel but significantly lighter, making it an essential material in aerospace and structural applications where every ounce counts in achieving lightweight design.
- Excellent Corrosion Resistance: Titanium is highly resistant to corrosion by seawater, chlorine, and even harsh chemicals, making it a choice metal in marine, medical, and chemical-processing applications.
- Biocompatibility: Titanium is a favored material for medical implants, joint replacements, and dental implants because it poses no toxicity and does not react adversely when exposed to human tissue.
- High Melting Point: Titanium has a melting point of 1,668°C (3,034°F) and so finds use in applications such as jet engines and power plants where high temperatures exist.
- Low Thermal Expansion: It exhibits very low thermal expansion, thereby maintaining dimensional stability across a wide range of temperatures —a beneficial property in precision engineering and aerospace design.
Titanium’s Superior Corrosion Resistance
Titanium, due to its exceptional corrosion resistance, holds a high status among metals. The corrosion resistance is owed to a thin, stable layer of oxide formed on its surface by the reaction of titanium with oxygen. This oxide layer acts as a protective coat on the metal surface, thereby obstructing any interaction between titanium and corrosive emanations. Studies have shown that titanium exhibits the best corrosion resistance in seawater, chlorine solutions, and acidic media. That is why it is chosen for marine, chemical processing, and medical applications. This resistance decreases maintenance costs and increases product life, giving titanium a competitive edge even under the harshest conditions.
Understanding Rust vs. Corrosion: The Scientific Distinction
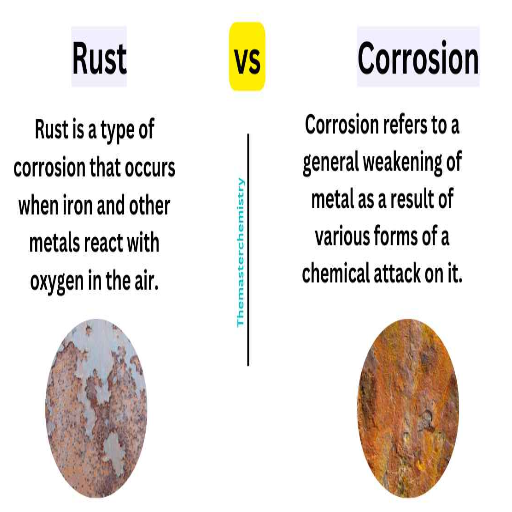
Rusting and corrosion, although forms of material degradation, do not have the exact causes or mechanisms underlying them. Rust refers to a reddish-brown iron oxide that forms when iron or steel comes into contact with oxygen and moisture. It is a particular form of corrosion that occurs only in iron-bearing materials. Corrosion, in contrast, is a more general term used to describe the chemical or electrochemical degradation of various materials, including metals, resulting from their interaction with the environment. Thus, rusting is a form of corrosion, but not all corrosion is necessarily rusting. Another example of corrosion would be the formation of aluminum’s protective oxide layer without rusting.
Defining Rust and Its Mechanism
Rust is the reddish-brown substance that appears on iron or steel when these materials are exposed to oxygen and moisture over time. The process that causes rust is oxidation: iron reacts with water and oxygen to form iron oxides. The primary compound bestowing rust is, in fact, hydrated iron(III) oxide. The reaction can be represented as follows:
[ 4Fe + 3O_2 + 6H_2O \rightarrow 4Fe(OH)_3]
This iron hydroxide can lose some water to yield red-brown rust.
Rusting is a many-step process that can be accelerated when saltwater or acidic conditions exist, as they act as electrolytes to increase iron’s reactivity. In normal circumstances, paints, galvanized zinc layers, or other coatings protect a metal from rusting by preventing oxygen and moisture from entering.
The Broader Concept of Corrosion
Corrosion is a natural process of gradual destruction of materials, usually metals, by chemical or electrochemical interactions with the environment. Corrosion ranges from the deterioration of rusty materials to the degradation of other materials, such as concrete, polymers, or ceramics, under various weather conditions. Corrosion is very frequently an industrial issue because industries—-such as oil and gas, marine, and construction—consider corrosion management so that structural failures and financial losses may be averted.
For example, one of the most searched online phrases is probably “how to prevent pipeline corrosion.” Cathodic protection, corrosion inhibitors, and specialized coatings are among the most commonly employed methods. With the emergence of new technologies, corrosion monitoring systems combined with AI-powered predictive analytics are becoming increasingly appealing due to their precision and cost-effectiveness. Thus, the broad view of corrosion emphasizes the need for advanced protective techniques to be applied by different sectors to ensure infrastructure that remains durable and safe on a much longer basis.
Oxidation Processes Across Different Metals
Oxidation processes vary across metals, with some forming protective oxide layers (e.g., aluminum) while others corrode (e.g., iron forming rust).
|
Key Point |
Iron |
Aluminum |
Copper |
Titanium |
Gold |
|---|---|---|---|---|---|
|
Oxidation Type |
Rust |
Protective |
Patina |
Oxide Layer |
Minimal |
|
Reaction |
Fe₂O₃ |
Al₂O₃ |
Cu₂CO₃ |
TiO₂ |
None |
|
Impact |
Weakens |
Protects |
Aesthetic |
Protects |
None |
|
Speed |
Fast |
Slow |
Moderate |
Very Slow |
None |
|
Environment |
Moisture |
Air |
Air/Sulfur |
Air |
None |
Titanium’s Unique Properties: The Foundation of Resistance
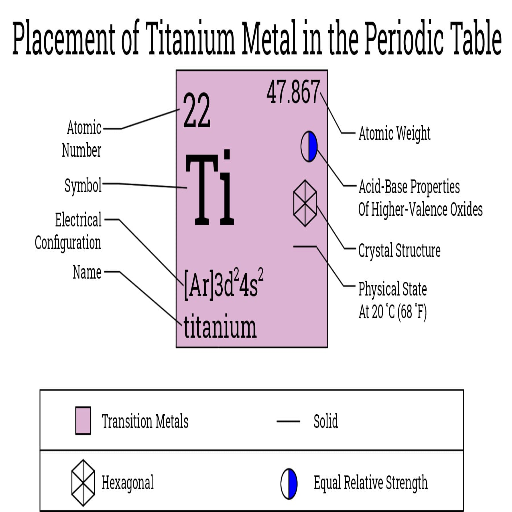
Titanium is so corrosion-resistant because it develops an oxide layer when exposed to oxygen. This thin layer is relatively stable and protects the bare metal from water, salt, and most chemicals. In fact, unlike many metals, the resistance of titanium is retained even in extreme conditions, such as high temperatures and a marine environment. These characteristics have thereby qualified it for use in the most critical applications in aerospace parts, medical implants, and marine engineering. The strength, lightness, and corrosion resistance that titanium offers have assured durable performance over long periods.
Physical Properties of Titanium
- Density: Titanium has a remarkably low density of approximately 4.5 g/cm³, which is almost exactly half that of steel, making it suitable for applications that demand strength coupled with a lightweight property.
- Melting Point: Titanium undergoes the melting process at temperatures of approximately 1,668°C (3,034°F), retaining its strength and stability under elevated-temperature conditions.
- Tensile Strength: Titanium, on the other hand, boasts some of the highest tensile strength, ranging from 240 MPa to well above 1,400 MPa, depending on the type of alloy, which makes it suitable for service in areas where exceptional performance is required.
- Thermal Conductivity: The thermal conductivity of titanium is considered relatively low, with a value of around 21.9 W/(m·K). However, this is sufficient for most industrial applications, particularly in minimizing thermal expansion.
- Corrosion Resistance: The natural oxide film on the surface indicates that titanium is highly resistant to corrosion by seawater, acids, and many chemical environments, thereby providing a long lifespan in harsh environments.
Chemical Stability and Reactivity
Titanium bonds to oxygen, granting it remarkable chemical stability. Thus, a fragile protective oxide film forms on the surface of titanium, protecting it from undergoing further chemical reactions. It exhibits high corrosion and oxidation resistance under normal conditions, as well as in aggressive environments. Usually, titanium is inert, but it can react somewhat under adverse conditions. At elevated temperatures, it reacts with halogens to produce titanium halides, while at high temperatures, it burns in pure nitrogen gas to produce titanium nitride.
Considering the scientific data, titanium’s chemical stability makes it an ideal choice for aerospace purposes, medical implants, and the chemical industry. This oxide layer provides a long lifetime for materials containing titanium and also reduces the potential for unwanted interactions. However, under certain extreme conditions, such as molten salts or fluorine gas, titanium begins to lose its passivity, highlighting the importance of undertaking material compatibility studies for such applications.
Formation and Benefits of the TiO₂ Layer
The naturally occurring titanium dioxide (TiO₂) layer arises on a titanium surface due to its considerable affinity toward oxygen. This passive oxide film is highly durable and self-regenerating, imparting significant advantages in various industries and biological applications. Some of the TiO₂ layer benefits are as follows:
- Corrosion Resistance: The TiO₂ barrier accelerates the prevention of reactions between the underlying titanium and environmental elements, such as water, air, and chemical compounds, thereby ensuring exceptional corrosion resistance for titanium even in marine or industrial environments.
- Biocompatibility: The TiO₂ layer is suitable for use in the medical field, for example, in implants, where its non-toxicity and inert nature minimize immune rejection and promote osseointegration.
- Enhanced Durability: This ensures that the protective layer extends the life of titanium by protecting it against wear and tear, as well as unfavorable conditions, making it an ideal choice for applications that require strength.
- Chemical Stability: The TiO₂ layer provides titanium with a protective shield capable of resisting powerful chemicals, making it very useful in the chemical processing industry, which utilizes strong acids and alkalis.
- Self-Healing Properties: The oxide layer resists damage and, upon scratching or exposure to oxygen, rapidly reforms to restore its protective properties, thus ensuring durability and usefulness over time.
These features can underscore the primary importance of the TiO₂ layer in guaranteeing titanium applications and enhancing its versatility.
The Passive Oxide Shield: How Titanium Protects Itself
Titanium’s protective layer is a thin, passive oxide shield of titanium dioxide (TiO₂), which naturally forms upon exposure to oxygen. These oxides form a veil, preventing further agitations from occurring between titanium and its environment. It also exhibits superior corrosion resistance, even under challenging conditions. Additionally, it can spontaneously repair any damage or scratches to ensure uninterrupted protection. This, therefore, is why titanium is so valuable across many fields due to its strength and dependability.
Instant Formation of the Protective Layer
As soon as the metal surface comes in contact with oxygen, the formation of the protective oxide layer on titanium proceeds almost instantaneously. Modern theories and research suggest that the formation of the layer is attributed to titanium’s high affinity for oxygen, which allows for the rapid development of a thin passivating layer of titanium dioxide (TiO₂). This self-forming and self-healing layer protects the metal from corrosion and degradation. If accidentally scratched or damaged, the layer almost instantly regenerates in the presence of oxygen, thus maintaining its protection. This property makes titanium highly sought after for applications in aerospace, medical implants, and marine engineering, where durability and corrosion resistance are required.
Self-Healing Mechanism of the Oxide Layer
Regarding the self-healing nature of the oxide layer on titanium, I would explain it as follows: a thin layer of titanium oxide (TiO₂) naturally deposits on the metal’s surface when it is exposed to oxygen. If the thin layer suffers any minor scratches or damage, it instantly regenerates in the presence of oxygen, thereby maintaining the surface’s protection. This ability to regenerate is what bestows titanium with extraordinary resistance against corrosion and wear.
Electrochemical Properties and Environmental Factors
Titanium exhibits exemplary electrochemical properties in various environments. These properties are present in multiple factors of the operational environment, which determine its efficiency in corrosion resistance and maintenance of structural integrity. Below are five principal electrochemical properties and influencing environmental factors:
- Oxide Layer Stability
In the case of titanium, its oxide layer (TiO₂) naturally forms and is highly stable across a range of pH levels, from acidic to alkaline.
- Corrosion Potential
Titanium possesses a high corrosion potential, making it poorly susceptible to corrosion even in aggressive environments such as seawater, chlorinated solutions, and oxidizing acids.
- Passivation
Titanium self-passivates, allowing for the rapid restoration of the oxide layer in the event of minor damage, thereby maintaining its integrity under various conditions.
- Temperature Influence
Titanium retains its corrosion resistance characteristics as long as the temperature does not exceed 600°F (315°C), making it suitable for industrial usage at high elevations.
- Environmental Contaminants
Titanium is resistant to corrosion caused by contaminants such as sulfides, nitrates, and chloride ions, which are commonly found in polluted or industrial atmospheres.
Environmental Performance: Where Titanium Excels and Struggles

Titanium is highly resistant to corrosion and exposure to high temperatures, and is used accordingly in marine, aerospace, and industrial applications, capable of withstanding saltwater, industrial chemicals, and extreme heat. However, titanium is prone to localized corrosion in waters with very high acidity or containing specific fluoride ions. These drawbacks should be taken into account when applying engineering and environmental considerations.
Excellent Resistance in Various Environments
When considering corrosion resistance and structural integrity, titanium stands out as unparalleled in various environments. As some new data reveal, titanium alloys have demonstrated extraordinary longevity in especially harsh conditions, such as seawater and industrial chemicals, surpassing the performance of ordinary materials like stainless steel. The resistance capabilities arise from an excellent passive oxide layer that heals itself upon damage, such as scratches, preventing further deterioration. Recent advancements in surface treatments and coatings have further enhanced corrosion resistance, solidifying its status as the material of choice for operations requiring an extremely durable material, such as marine engineering, medical implants, and aerospace construction.
Challenging Conditions and Limitations
Challenging situations and limitations are imposed on titanium, depending on the type of specialized application considered. Below are several significant limitations related to titanium:
- High Cost
Titanium costs much more to make than other metals, such as steel and aluminum. This exorbitant cost is due to its complex extraction and refining process, utilizing energy-intensive methods such as the Kroll process.
- Difficulty in Machining
Its strength and low thermal conductivity tend to cause excessive tool wear and overheating during machining. This often requires special tools and techniques, hence more machining time and expense.
- Susceptibility to Galling
When two titanium parts are in sliding contact, there is the risk of galling. This adhesive wear results in material transfer and damage to the surface, potentially affecting the component’s performance over time.
- Limited Performance in High Temperatures
Titanium, by reputation, boasts a very high strength-to-weight ratio; however, its mechanical properties diminish at elevated temperatures, typically above 800°F (427°C). This consequently makes it unsuitable for some high-temperature applications such as turbine blades of jet engines.
- Hydrogen Embrittlement Risks
Titanium can absorb hydrogen in specific environments and undergo hydrogen embrittlement. This phenomenon decreases ductility and toughness and could cause the untimely failure of materials in really critical applications.
Critical Failure Conditions and Stress-Corrosion Cracking
The bases for stress corrosion cracks in titanium alloys are particularly unique. Traditionally, tensile stress and a corrosive environment, combined with one of the halides (chloride, fluoride, or bromide ions), are inhibitors that can cause stress corrosion cracking and induce sudden, unexpected failure of an otherwise robust material. Titanium is considered a passive metal with very high resistance to corrosion in general environments. Still, this resistance can be compromised under specific stress and temperature conditions when the protective oxide layer is damaged.
Recent studies have shown that the titanium SCC phenomenon depends on alloy composition, the magnitude of stress, and the cyclic nature of loading. For example, cases of SCC in chlorinated environments or under prolonged seawater attack on titanium components have been reported from the aerospace and marine industries. To reduce these risks, proper material selection, controlled stress environments, and regular maintenance of protective coatings are necessary. Research into surface treatments and alloying to enhance resistance and reduce susceptibility to SCC under critical service conditions is underway.
Reference Sources
- Princeton University: Corrosion – Titanium – Explains how titanium resists corrosion by forming a passivating oxide layer.
- University of Toledo: Titanium – Highlights titanium’s exceptional corrosion resistance, even in extreme environments.
- Missouri University of Science and Technology: Corrosion Products of Titanium – Discusses specific conditions under which titanium may corrode.
- U.S. Nuclear Regulatory Commission: Corrosion Resistance of Titanium – Details titanium’s corrosion resistance in various environments, including moist and dry chlorine.
Frequently Asked Questions (FAQs)
Q: What is the rust resistance of titanium?
A: Titanium is known for its exceptional rust resistance. Unlike common metals such as steel and aluminum, titanium does not rust in the same way. Instead, when exposed to oxygen, titanium forms a protective layer of titanium oxide on its surface, which prevents further corrosion and protects the pure titanium beneath.
Q: Can titanium corrode under certain conditions?
A: While titanium is generally resistant to rust and corrosion, it can corrode in specific environments, such as in the presence of hot nitric acid or salt water. In these cases, titanium can be susceptible to localized forms of corrosion, such as pitting and crevice corrosion.
Q: How does titanium react with oxygen?
A: Titanium reacts with oxygen to form a layer of titanium oxide. This oxide layer is what makes titanium resistant to rust and corrosion, effectively protecting the underlying titanium metal from degradation in various environments.
Q: Is titanium more resistant to rust than other metals?
A: Yes, titanium is more resistant to rust than many other metals, including steel and aluminum. Its unique properties allow it to withstand corrosion in a wide range of environments, making it ideal for use in chemical plants and marine applications.
Q: What are the properties of titanium that prevent rust?
A: The properties of titanium that prevent rust include its ability to form a protective oxide layer and its hardness. This layer protects the pure titanium from contact with air and moisture, which are common catalysts for rusting in other metals.
Q: What is the role of titanium grades in corrosion resistance?
A: Titanium comes in various grades, each with different alloying elements added to enhance its properties. Some titanium grades offer improved resistance to corrosion, particularly in harsh environments, making them suitable for specialized applications.
Q: What happens to titanium when it is exposed to seawater?
A: When titanium is exposed to seawater, it generally resists rust and corrosion due to its protective oxide layer. However, under certain conditions, such as high salinity or temperature variations, localized corrosion can occur; nevertheless, it remains significantly better than many other metals.
Q: How does titanium compare to titanium alloys in terms of rust resistance?
A: Titanium alloys may offer varying degrees of rust resistance depending on the alloying elements used, such as vanadium or chromium. Generally, titanium alloys exhibit a high level of corrosion resistance; however, their specific properties can vary depending on the composition and intended application.
Q: Where can I find titanium products known for rust resistance?
A: You can find titanium products that are known for rust resistance in various industries, including aerospace, medical, and marine applications. These products are often made with titanium due to its superior properties, including resistance to corrosion and rust.

