The alloy steel has performed exceptionally well in terms of its tenacity, adaptability, and ability to withstand the rigors of construction, production, and mechanical applications. It is as durable as it is possible and then some. Nonetheless, as with any material left in the atmosphere, a pertinent question has been raised: Does steel alloy, over time, rust? The ability of an alloy steel to resist corrosion on its outer surface is of great importance to producers and consumers, as it directly affects the service life of the products and structures. This post will describe how alloy steel is crafted and will contrast its ability to resist corrosion with that of alternative materials, as well as enumerate the critical parameters that enhance its performance in severe operational environments. If the notion of alloy steel from the very beginning up to its prolonged use has bothered you, you will find the article helpful.
Introduction to Alloy Steel and Rust

Composite metal is a combination of steel and other metals that may incorporate chrome, nickel, or sometimes molybdenum to increase the strength and reduce vulnerability to the weather. The corrosion resistance is generally a factor determined by the level of inclusion of different metals, with chromium being the most active and forming a thin film of metal oxide over the steel surface. This sheath serves as the cutoff, blocking the penetration of moisture and air to the base material. It is pretty apparent that, compared to carbon steel, alloy steel outperforms it in terms of rust prevention, making it suitable for environments with harsh conditions or those that are wet.
What is Alloy Steel?
Alloy steel is a type of steel that depends on the presence of additional elements in its composition in considerable quantities. Namely, suppose it’s chromium, nickel, or other elements, such as cracks, with obviously high carbon, manganese, and vanadium content. In that case, these additions are incorporated into the alloy to enhance its mechanical properties, including hardness, strength, toughness, wear resistance, and corrosion resistance. It is estimated that alloy steel accounts for nearly twenty-five percent of all other types of steel used in construction, automotive, aerospace, and energy-related industries, due to its numerous factors and various applications. Furthermore, the chemical components in alloy steel are likely to differ depending on its intended use, and therefore, different solutions can be developed. Furthermore, new trends in science have enabled the creation of significantly more effective alloy steels, which are of higher quality, greater durability, and more environmentally friendly due to their recyclability.
How Does Alloy Steel Compare to Other Metals?
Toughing it out with enhanced mechanical features and usage, the alloy bears a close resemblance to other metals. Regarding plain carbon steel, alloy steel offers enhanced physical characteristics, including improved strength, hardness, and resistance to wear and corrosion. Therefore, it is considered a preferable material for highly demanding fields, such as the automotive, construction, and aerospace industries, among others. Looking at aluminum, it is a rigid material that is very light, but it lags in tensile strength; hence, structural members with a service life are often made from alloy steel.
Moreover, unlike stainless steel, alloy steel allows for more flexibility in adjusting its mechanical properties based on its chemical composition, making it also more rational for specific applications. The low-alloy phase of the structural literature, which includes elements such as chromium, molybdenum, nickel, and their alternatives, copper and zinc, offers higher corrosion resistance in many aging environments. Innovative technology that can also be modified and reused, and in the broader context, sustainability and recyclability concerns. This, in conjunction with the performance-targeted design, ensures the product stays a step ahead in the modern materials market.
Understanding Rust and Corrosion
Rust is a specific type of corrosion that affects iron and its alloys, whereas corrosion is a broader process that degrades various materials through chemical or electrochemical reactions.
|
Parameter |
Corrosion |
Rust |
|---|---|---|
|
Definition |
Material degradation |
Oxidation of iron |
|
Materials Affected |
Metals, ceramics, polymers |
Iron and its alloys |
|
Conditions |
Air, chemicals |
Air and moisture |
|
Products |
Oxides, salts |
Iron oxide |
|
Appearance |
Varies (e.g., green, blue) |
Red-brown flakes |
Corrosion Resistance of Alloy Steel

Alloy steels are known to mitigate the adverse effects of corrosion, primarily due to the presence of chemically active elements, such as chromium, within the alloy. This chromium forms an oxide layer over the steel, which hampers water and oxygen from getting into contact with the steel. Consequently, the most significant oxidative degrading factors—moisture and oxygen — are successfully restrained. However, the degree of corrosion resistance also varies due to the steel grade, with higher chromium content providing greater efficiency. In conclusion, alloy steel is well-suited for applications that require extensive service in severe conditions.
Why Alloy Steel is More Resistant to Rust
It is generally believed that alloy steels have greater resistance to oxidation processes. This is due to the large percentage of chromium in it, which combines with oxygen in the environment to develop a thin, self-healing oxide layer called chromium oxide. This layer acts as a protective coating, retarding further oxidation and the penetration of moisture and other corrosive substances into the material. Furthermore, recent designs of alloy steels incorporate reinforcing additives such as nickel, molybdenum, and manganese to enhance the material’s resistance to harsh environments, including seawater and aggressive chemicals. The advancement in the alloy steel field also highlights the significance of effective processing, including heating and coatings, which shield alloy steel from corrosion processes that can be considered adequate. The aforementioned factors, together with structure, determine the alloy steel configuration that is attractive for all the most demanding environments, including the shipbuilding, construction, and other industrial sectors where high wear resistance is required.
Key Alloying Elements: Chromium, Nickel, and Molybdenum
Chromium enhances corrosion resistance and hardness, nickel improves toughness and ductility, and molybdenum increases strength, hardenability, and resistance to pitting.
|
Parameter |
Chromium |
Nickel |
Molybdenum |
|---|---|---|---|
|
Key Role |
Corrosion resistance |
Toughness, ductility |
Strength, hardenability |
|
Corrosion |
Forms a protective layer |
Acid resistance |
Pitting resistance |
|
Strength |
Increases hardness |
Enhances impact strength |
Improves tensile strength |
|
Applications |
Stainless steel |
Austenitic steels |
Tool steels, for marine use |
The Role of Oxidation in Metal Corrosion
During the degradation process, the corrosion of metals is also significantly enhanced by oxidation, which plays a mediating role in chemical processes between metals in a relatively humid environment with oxygen/moisture. For example, when material and water are provided to a surface containing metal, such as iron or steel, exposure to elements, especially oxygen and water, causes an electrochemical oxidation of iron. This process transforms the most common form of metallic iron (Fe) into rust. This form of corrosion has numerous drawbacks, as it not only weakens the substrate’s structure but also imposes a significant financial burden on the various sectors that rely on metal.
Recent scientific conclusions indicate that advances in anti-oxidation treatment – coatings and inhibitors – are now at the stage of practical implementation. Thus, coatings with zinc and other metals, as well as inhibitors, have additional physical barriers that prevent oxygen and moisture from contacting the metal. Yet, in the case of alloys containing a cliff electrode potential element, a chromium oxide layer forms around the flakes, acting as a physical barrier to further corrosion. This demonstrates progress in material science in counteracting this ongoing problem. The acquisition of knowledge and comprehension of the concept of oxidation, along with its in-depth understanding, has immense critical applications in the development of rapid solutions in various fields, including construction, transportation, and cloud computing.
Factors Contributing to Rust Formation
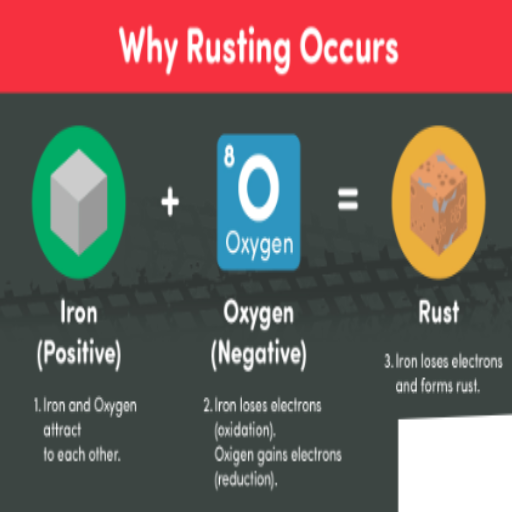
The reaction between iron, water, and oxygen primarily causes rust. There are several fundamental issues when it comes to Rust development, including:
- Moisture: When a material is exposed to or submerged in water for a prolonged period, or even in a humid environment, it can cause the material to rust quickly.
- Oxygen: Ruin on metallic surfaces requires that the air contain some amount of oxygen.
- Salt: Sea water, particularly when it is on the seaside or during the winter season, increases the rate of rusting by containing electrolytes, which promote the reaction.
- Temperature: Rusting is hastened in warmer wetter climates, but prolonged in cold dry climates.
- Pollutants: These are gases that are causing air pollution by dissolving in precipitating rainwater, and making it acidic, which, in the presence of adequate moisture, enhances rusting.
To minimize the effect of these factors, which cause rust, it is vital to apply protective coatings or to control the environmental conditions.
Environmental Conditions Affecting Rust
Environmental changes primarily influence rusting; therefore, understanding these changes is crucial for its prevention. The most recent trends and research indicate that rusting is always severe in the vicinity of the ocean. This is because salt is present in the environment and acts as a conductive medium, significantly enhancing moisture-induced corrosion. As if this is not enough, the existence of an urban population, which is exposed to high levels of pollutants, including sulfur, leads to acid rains that are even more destructive to metallic materials.
One thing is for sure: how can one withstand such effects of unfavorable circumstances? There are solutions suggested/encouraged, such as taking preventive measures like utilizing materials resistant to rust, like stainless steel, and using paints or covers that inhibit corrosion. Additionally, ensuring there is always enough ventilation to prevent elevated humidity is also recommended. All these have been recognized as the most productive means. Moreover, regular cleaning and servicing help to contain the extent of environmental influence, preventing rusting.
Impact of Improper Maintenance on Alloy Steel
Lack of proper maintenance of alloy steels risks causing several adverse effects. The primary result of this is an extensive decline in the strength and efficiency of the material. Alloy steel in its limitless extension suffers from more corrosive tendencies when compared with mild steel, especially more so when the environment is temperate, humid, or after being harshly acted upon by chemical and salty substances. The corrosion will be significantly accelerated in the presence of these environmental factors, especially in the absence of regular maintenance, as multiple surveys indicate. Any dirt present can form residues and deteriorate corrosion by adsorbing moisture and other impurities onto the surface of the material. The aforementioned enhancement in damage potential reduces the load-bearing capacity of alloy steel. It is, in fact, responsible for numerous mishaps when the material is used in critical applications, such as construction, transportation, and industrial machinery.
Additionally, poor care of the metal may cause the failure of the paints or coatings that were previously applied to the metal. The anticorrosive agents were effective when the oxide layer was intact, but upon destruction of the oxide layer, the material rusted very quickly. It is vital to carry out routine, structured examinations and implement corrective measures, such as re-applying protective coatings, controlling the impact of locally sourced aggressive aspects, and immediately rectifying any signs of degradation or mechanical damage, to increase the useful life of the alloyed steel.
Types of Rust: What to Look Out For
Rust is formulated differently, depending on the conditions and the type of material being exposed. The following are five common types of rust, their properties, and what to look out for:
- Uniform Rust
This is the most common type of rust, characterized by its uniform appearance on the surface of the metallic material. In many instances, it seems to be a result of prolonged exposure to a humid or moist atmosphere. Regular checking is a key factor in controlling and preventing general rusting.
- Pitting Corrosion
This form of corrosion develops when the metal surface comes into contact with a highly corrosive substance, such as chlorides, at localized points. This produces small holes in the metal, which tend to be relatively small in radius, and where cutting may take place over time.
- Crevice Corrosion
This type of rust is typically associated with concealed areas, such as joints, overlaps, or gaps that trap moisture, thereby facilitating the formation of crevices. Inspection and correction of these problematic areas, such as restoring them to facilitate drainage, are remedies that significantly minimize the likelihood of crest corrosion.
- Galvanic Corrosion
Occurs when two different materials come in contact with each other under an electrolytic solution, such as water. The vulnerable or anode material is the one that dwells in the electrochemical series. Prevention of galvanic corrosion is obtained by using non-metallic isolating layers between the alloys.
- Flash Rust
Flash rusting occurs rapidly when bare metal is exposed to water for a very short period. It is characteristic of wet indoor or outdoor construction environments. The rust should not be allowed to advance, and this can only be achieved through prompt and appropriate drying and protection.
Knowing the types of rust and addressing the problems promptly can help prevent significant damage and prolong the lifespan of steel components.
Types of Alloy Steels and Their Applications

- Low-Alloy Steels
Regarding low-alloy steels, it is adequate to explain that small amounts of elements, such as manganese, nickel, or chromium, are present in these materials. Due to their robustness and resistance properties, they are utilized in structural works, pipeline construction, and vehicle components.
- High-Alloy Steels
High-alloy steels are modified to have higher values of constituent elements, such as chromium and nickel. This permits them to be used in corrosive environments and is well-suited for particular products, such as cookware, medical devices, and other items that involve handling various levels of chemicals.
- Tool Steels
Tool steels are used for making tools and dies and are hardened to withstand higher temperatures and impacts; thus, they are preferable in cutting, stamping, and machining processes.
- Microalloyed Steels
Microalloyed steels are strengthened by small amounts of information added, such as the residue of Vanadium or Titanium, which increases their strength and adds very little total weight or mass. These are found in many applications, including transportation and structural components.
This appreciation for the nature of intention, as well as the extent of certain steel types, is necessary to choose the most suitable alloy steel for a diverse range of industrial and commercial applications.
Common Types of Alloy Steels
1. Stainless Steels
Stainless steel is a class of metal alloys known for their high corrosion resistance. This type of steel is composed of at least 10 percent chromium, which usually reacts with oxygen to form an invisible protective film on the steel surface. These are used in numerous areas, including kitchen applications, medical devices, building and construction, and the chemical industry. They can withstand a lot of wear and tear, and their shiny surface retains its original state. Types such as Austenitic, Martensitic, and Ferritic stainless steels are adapted to endure the features that result from their structures.
2. Tool Steels
Tool steels are manufactured to be strong, complex, and resistant to heat, making them the most suitable for cutting, forming, and molding. These steels are commonly imbued with tungsten, molybdenum, vanadium, and chromium. They are also used in the fabrication of tools, dies, and molds that cannot change their properties when exposed to wear and extreme temperatures.
Applying AIC technology or simply manipulating the chemical composition and physical structure of steel improves elongation and tensile strength. Of course, alloy steels are still the most preferred and desired materials, as they can fill the gaps in industries such as manufacturing.
Applications in Various Industries
- Automotive Industry
In the automobile industry, alloy steels are used in the manufacturing of almost all mechanical components, such as gears, crankshafts, and connecting rods, due to their superior mechanical properties, primarily high strength and the ability to withstand wear and tear. These substances promise durability and functionality by performing well under severe loading and high-temperature conditions.
- Aerospace Industry
Parts such as landing gears, turbines, and load-bearing structures utilize the robustness of alloy steels for fatigue resistance and thermal shock tolerance, which are crucial in the industry. These features help ensure safety and increase operational efficiency in the aerospace sector.
- Construction Industry
A wide range of obstacles, including the construction of bridges, the erection of buildings, and the improvement of infrastructure, utilize alloy steels as well; every profession embraces alloy, with its use in bending, shear resistance, and corrosion resistance. By allowing for the use of lovingly crafted structural members, alloy steels enable the long-term operability and predictability of the products.
- Oil and Gas Industry
Alloy steels are utilized in drilling tools, pipelines, and pressure vessel systems because the materials do not degrade when used under harsh conditions. The pressure and temperature resistance requirements of this sector necessitate the use of alloy steels.
- Energy Sector
Energy production, specifically power plants, utilizes alloy steels in the manufacture of critical components, such as boilers, turbines, and heat exchangers, to help manage extreme temperatures and pressures. These features are typical of low-alloy steel if the alloy is within the boundaries of such steels.
Comparing Alloy Steel to Carbon Steel and Stainless Steel
Alloy steel offers high strength and wear resistance, whereas carbon steel is cost-effective and has a higher hardness. Stainless steel excels in corrosion resistance and aesthetics.
| Parameter | Alloy Steel | Carbon Steel | Stainless Steel |
|---|---|---|---|
|
Strength |
High |
Moderate |
Moderate |
|
Corrosion |
Moderate |
Low |
High |
|
Cost |
Moderate |
Low |
High |
|
Applications |
Construction, tools |
Structural, tools |
Medical, kitchenware |
|
Aesthetics |
Moderate |
Low |
High |
Preventing Rust in Alloy Steel

To prevent corrosion on the alloy steel, exposure to moisture and corrosive environments should be minimized as much as possible. Painting and coating with oil or serviceable corrosion protection can offer particularly effective defense against corrosion. Even so, there is value in placing the alloy steel in a humid, controlled, secluded area that is dry to reduce the chances of rust creation. It is also effective to inspect the steel regularly for maintenance purposes, including cleaning and checking for signs of corrosion. Adding to its alloy strength in such conditions, the alloy with a high chromium content also enhances its resistance to rust.
Protective Coatings and Their Benefits
The importance of protective coatings cannot be overstated, particularly when it comes to materials like metals. They are protectors against corrosion, wear, and tear on a large scale. Moreover, with the proper coating material, it may outlive available maintenance schedules and enhance cost savings over time. Herein lies an elaboration of five standard protective coatings with the associated advantages:
- Epoxy Coatings
Epoxy coatings are long-lasting and provide adequate protection against chemicals, water, and abrasion. This is due to their keen use in industrial installations as well as buildings of steel. According to various analyses, epoxy coatings can help save material by as much as 10 years under harsh conditions.
- Polyurethane Coatings
The properties of polyurethane coatings include their extreme flexibility and ability to withstand UV light. Polyurethane coatings are mainly used for outdoor applications. They prevent the materials from hardening when used outside in the sun, and they also have a shiny finish. The data indicate that such coatings maintain their appearance and functionality for 5-8 years with little to no deterioration.
- Zinc-Rich Coatings
Zinc-rich coatings, commonly used as a primary coat for steel systems, offer long-term corrosion protection. The environment reacts with the zinc, causing it to corrode, thus protecting the steel from rust formation. As such, they are broadly used in the marine and other industries, and they can extend the life of the material by 15-25 years in specific cases.
- Ceramic Coatings
This type of heat-resistant material coats the steel, protecting the surface from both high temperatures and abrasive actions. It finds applications in cars and planes, most of all. The evidence indicates that they can survive temperatures of up to 1830°F and preserve their qualities.
- Powder Coatings
When powder coatings are applied, a very durable finish resistant to environmental factors and a composite, environment-friendly finish is obtained, which is subject to a high degree of chipping, fading, and scratching. They are compounds of different colors and textures that are useful for a customer’s decorative purposes. According to the research, they may last under such conditions for no more than 15-20 years if they are not used properly.
All these coatings are necessary as they will contribute to increasing the usage and changing the dynamics of given materials across all industries. The choice and application of coatings are crucial, as they enhance the materials’ strength and functionality over an extended period.
Establishing Proper Cleaning and Maintenance Routines
Before proceeding, I assess the materials or surfaces and determine the necessary ingredients, techniques, and equipment required for cleaning and maintenance. I only use those cleaning agents that are not harmful, and I make it a point to clean regularly so that dirt or contaminants do not accumulate. Another issue that cannot be ignored is the environmental conditions, such as humidity and temperature, which can have profound implications for the maintenance program in place. Through this, it will be easy to keep everything in order and ensure the most appropriate measures are taken to preserve the materials, allowing them to last as long as possible.
Choosing the Right Grade of Alloy Steel for Specific Environments
In certain areas, more advanced materials with high strength are necessary for specific applications to ensure enhanced resistance and superior performance. Such materials are called steel, with its many types, which are created to meet the environmental conditions specified for that steel. For applications where the scale of the effect is relatively high in terms of material deterioration, its resistance to corrosion and load fatigue must surpass the limits covered by carbon and low-alloy steels. For example in order to resist the extreme conditions of high temperature such as those in power generation and chemical, specific steels such as T91 and P91 are considered. Corrosion is one of the factors that contribute to materials quickly subjected to heat, hence the need for steels like T91 and P91. Similarly, in cases of service temperature, the application of solutions such as 4130 and 4340, which are low-alloy high-strength steels, is recommended.
In applications such as construction, transportation, and leisure, it is evident that the selection of materials is continually evolving. This is evident in modern selection criteria, as methods are becoming more sophisticated and include computer-aided calculations, which assist in determining the grade of alloy steel within the acceptable limits of operational temperatures. These advanced tools facilitate informed decision-making, taking into account the latest technical developments and international norms in both low- and high-temperature conditions.
Reference Sources
-
ScienceDirect: Corrosion resistance and mechanical properties of low-alloy steel – Discusses the role of rust density and bonding performance in corrosion protection.
-
IOP Science: Corrosion of Carbon Steel and Alloy Steel – Explores corrosion mechanisms and their impact on alloy design and resistance.
-
PubMed Central (PMC): Rust Formation Mechanism on Low Alloy Steels – Examines the chemical states of iron in rust layers on low alloy steels.
Frequently Asked Questions (FAQs)
Q: What is alloy steel, and how does it relate to rust?
A: Alloy steel is a type of steel that consists of iron and other elements, such as chromium, nickel, and molybdenum, which enhance its properties. While alloy steel can be more resistant to rust compared to carbon steel, it is not entirely immune to rust formation. Factors such as the presence of moisture, oxygen in the air, and the amount of iron present can lead to corrosion and rust if not correctly managed.
Q: What factors contribute to the rusting of alloy steel?
A: The rusting of alloy steel is influenced by several factors, including the presence of water, oxygen, and the specific alloying elements used. For instance, a higher chromium content can improve corrosion resistance by forming a protective layer on the steel surface, while the presence of moisture can accelerate the rusting process.
Q: Is alloy steel rust-resistant?
A: Alloy steel can be considered rust-resistant depending on its composition. Alloys that include elements such as chromium and nickel can offer improved resistance to corrosion, forming a protective layer that helps prevent rust or tarnish. However, without proper maintenance and protective coatings, such as galvanization, alloy steel may still rust.
Q: What is galvanization, and how does it help prevent rust?
A: Galvanization is a process that involves coating steel components with a thin layer of zinc. This layer acts as a barrier, preventing oxygen and water from reaching the steel surface, thus significantly reducing the likelihood of rust formation. Galvanized steel is often used in environments where corrosion is a concern.
Q: How does the presence of moisture affect alloy steel?
A: The presence of moisture is a critical factor in the rusting process. When alloy steel is exposed to water and oxygen, it can form iron oxide, commonly known as rust. To prevent this, it’s essential to keep alloy steel dry and consider using corrosion-resistant coatings.
Q: Are there metals that are considered rust-proof?
A: While no metal is entirely rust-proof, some materials, like stainless steel and aluminum, exhibit excellent corrosion resistance. Stainless steel, for example, is an alloy that includes a high chromium content, which forms a layer of chromium oxide that protects against rust and tarnish, making it a popular choice for applications requiring durability.
Q: What is the difference between alloy steel and stainless steel?
A: Alloy steel and stainless steel are both types of steel that incorporate various elements to enhance their properties. However, stainless steel is specifically designed to resist corrosion due to its high chromium content. In contrast, alloy steel may not offer the same level of corrosion resistance unless it contains similar alloying elements.
Q: How can rust prevention be achieved for alloy steel?
A: Rust prevention for alloy steel can be achieved through several methods, including applying protective coatings, such as paint or galvanization, and ensuring proper maintenance. Regular inspections and cleaning can also help to remove any moisture or contaminants that may lead to corrosion and rust formation.

