In other words: Nickel, versatile and essential, is an important element in metals and modern technologies. It is considered a strong element that resists corrosion and is magnetic. Being so, it acts as the backbone of industries such as manufacturing, energy, and electronics. But what properties make nickel unique? A lot of attention is usually given to its density and peculiar characteristics in materials science. This article focuses on the properties of nickel and its place among the metals. If you consider yourself an inquisitive learner or an expert in the field, you will gain much knowledge concerning the interesting world of nickel and its applications.”
Introductions to Nickel and Its Density

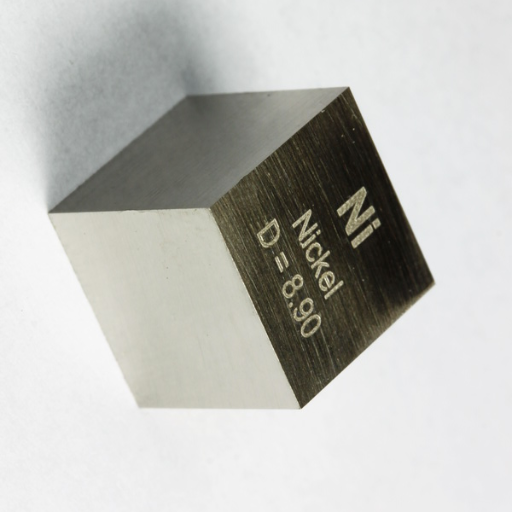
Nickel is important uniquely in that it possesses qualities of strength, corrosion resistance, and versatility which make it one of the quintessential metals needed in an array of industries. With a density of 8.90 g/cm³, nickel is compact and durable, allowing its widespread use in alloys such as stainless steel, to bring about strength and corrosion resistance. It also finds predominant use in electronics and industrial applications as it can withstand high temperatures-Batteries, turbines, and chemical equipment. And that, in essence, is why this metal is playing a paramount role in contemporary material science; these characteristics endorse nickel in accommodating the needs of different advanced technologies.
Importance of Nickel in Industry
Due to its highly versatile properties that impart the modern technologies, nickel stands to benefit several industries. The leading application of nickel in the industry is its usage for making stainless steel, which uses some 68% of all nickel consumption all over the world. Stainless steel with nickel as a key element remains corrosion-resistant and durable, with applications in construction and architecture and household appliances.
After stainless steel, nickel is life-giving in batteries, mainly Ni technology affiliated with batteries for charging electric vehicles (EVs). Nickel-rich batteries have a considerable chunk in the EV market, most notably in Nickel-Cobalt-Aluminum (NCA) and Nickel-Manganese-Cobalt (NMC) chemistries. According to the most recent reports, nickel demand for batteries faces an expected meteoric rise of 30-40% per annum with transition towards sustainable transportation gathering pace.
Nickel alloys are of principal importance in applications, such as jet engines and gas turbines, which require materials capable of resisting extreme temperatures and pressures. Nickel alloying with chromium and molybdenum adds to mechanical strength and environmental resistivity. Nickel in the energy sector is also utilized in desalination plants, petrochemical installations, and renewable energy applications.
New recycling methods have furtherly contributed to geasing up nickel’s industrial importance, according to which 68% of all stainless steel scrap and other nickel-bearing materials go into recycling. This fact of sustaining brings to light the engagement of nickel, whom has also become vital for technological growth as well as conservation. With worldwide nickel production of nearly 3 million metric tons yearly, it is bound to carve a deep niche into shaping industries and promoting present-day innovations.
Nickel Density: A Short Insight
Compared to one cm³ space, surrounded by the atmosphere at room temperature, nickel has an approximate weight of about 8.908 g, ranking it among the more dense natural elements. This property, being so vital, sets the tone for the numerous uses nickel has in so far as metallurgy and materials science go. For example, nickel’s weight brings about advantages by allowing it to impart mechanical stability to construction and manufacturing preparations by way of sturdy alloys such as stainless steel.
Its density also lends these qualities of heat stroke and corrosion resistance. These are needed in harsh applications such as those in chemical plants, aerospace, and marine applications, where durability and reliability under extreme conditions are a must. This practice for recycling, combined with the weight, greatly boosts its worth in supporting sustainable material profiles for the present dynamic industries.
Significance of Density in Material Sciences
Density serves as a focal point from the point of view of material sciences that directly affect a material’s mechanical, thermal, and chemical features. Density, being the mass per unit volume of a material, upholds how a material works when in a stressed state and thus, its applicability in all engineering and industrial settings.
By way of example, in the dense substances, tungsten, with a density of 19.25 g/cm³, and lead, with 11.34 g/cm³, are selected when resistance and energy absorbing are needed for radiation shielding or for ballast weight. Conversely, less dense substances, such as aluminum (2.7 g/cm³) and titanium (4.5 g/cm³), are useful in aerospace and automobile industries to keep weight down without compromising on structural strength.
Density affects thermal properties too. High-density substances such as copper (8.96 g/cm³) conduct heat well into electronics and cooling systems. On the other hand, low-density materials like polyurethane foam are used as insulators due to their low thermal conductivity.
Recent studies accentuate density in pushing further to achieve better materials that cater to specific needs. Innovations in composite materials give insight into how the disparate densities of materials can be combined for optimum performance. For instance, carbon fiber-reinforced polymer, with a density around 1.6 g/cm³, is distinguished by a high strength-to-weight ratio, a feature dearly valued in the industries on the cutting edge.
With this understanding and perspective of density, scientists, and engineers can give a material that caters to the current technologies in an environmentally friendly way.
Scientific Properties of Nickel

Nickel is a silver-white metal of good durability and resistance to corrosion. With an atomic number of 28, nickel is a transition metal in the periodic table. Nickel is extensively used in alloys, for example, stainless steel, to improve their strength and corrosion resistance. Also, nickel is ferromagnetic at room temperature and displays good thermal and electrical conductivity, which can be exploited for several industrial purposes.
Atomic Structure and Density
Nickel has 28 protons in its nucleus and is therefore the 28th element in the chemical series. It has an atomic mass of approximately 58.69 atomic mass units (amu). The element is found mostly in one of its stable isotopes-Nickel-58-in contrast to natural nickel, which is itself divided into five isotopes, namely, Nickel-58, Nickel-60, and Nickel-62. The electron distribution of nickel is [Ar] 3d⁸ 4s², which places the metal in the d-block of the periodic table.
11.54 Building on the high-value density, nickel’s density stands at 8.908 g/cm³ at room temperature, signifying a higher density among the metals, an advantageous property for industrial use associated with the metal’s strength and rigidity. Nickel melts at a higher temperature of 1,455 °C (2,651 °F), whereas it boils at 2,913 °C (5,275 °F), thereby pointing towards its usefulness as a thermally stable and highly adaptable metal at elevated temperatures. Therefore, it finds application in turbine blades and heat-resistant alloys.
Oxidation States of Nickel
Nickel forms mainly oxidation states +2 and +3; however, +2 is the most commonly encountered oxidation state in Ni-containing compounds. The +2 oxidation state, written as Ni²⁺, is very important in nickel chemistry due to its formation of relatively stable compounds such as nickel sulfate (NiSO₄) and nickel chloride (NiCl₂), while the +3 state is much less common and is only found in certain compounds such as nickel(III) oxide (Ni₂O₃), mainly in specialized chemical contexts.
This variability in oxidation states allows nickel to be employed in batteries, in particular nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) rechargeable batteries. NiCd batteries are recognized for their long life, while NiMH ones are appreciated for having greater energy density and, thus, extensively considered for the environment. Furthermore, nickel is credited for increasing the strength of modern lithium-ion batteries in the form of nickel-cobalt-manganese (NCM) cathodes, which are immensely favored for electric vehicles and portable electronics.
Isotopes of Nickel and Their Properties
Nickel has five naturally occurring stable isotopes, which include Nickel-58, Nickel-60, Nickel-61, Nickel-62, and Nickel-64. Among them, Nickel-58 is the most abundant, constituting roughly 68.27% of naturally occurring nickel. It is followed by Nickel-60 at 26.10%, Nickel-61 and Nickel-62 on a significant drop at approximately 1.14% and 3.63%, respectively, and finally, Nickel-64 with just about 0.93% in nature.
With nuclear properties differing from each other, the various nickel isotopes are used widely in scientific as well as industrial applications. For example, Nickel-62 has an exceptionally high binding energy per nucleon, rendering it extremely stable. Moreover, Nickel isotopes play a crucial role in astrophysical studies concerning element formation during supernova explosions, wherein isotopes such as Nickel-56 decay to form iron.
Artificial isotopes of nickel have also been produced, namely Nickel-57 and Nickel-59, used in tracer studies and radiology. Nickel-59 has relevance in the study of corrosion in metals and long-term geological processes because of its long half-life of 76,000 years. An understanding of these isotopes not only enriches the field of nuclear chemistry but also contributes towards new insights into a plethora of practical applications from environmental research into material sciences.
Applications of Nickel in Industrial Sectors

Due to its versatility and singular properties, nickel is widely applied throughout many industries. For example, the manufacturing sector uses nickel for producing stainless steel and corrosion-resistant alloys. The energy sector utilizes nickel for producing batteries, particularly for rechargeable lithium-ion batteries intended to power electric vehicles and portable electronic gadgets. Furthermore, for very high-temperature resistance under extreme conditions, aerospace industries consider nickel a critical component in superalloys. In turn, these diversified applications make nickel a truly invaluable commodity in modern industrial and technological advances.
Nickel in Alloys and Its Merits
Nickel is key to the production of high-performance alloys that play essential roles in many industrial applications. Primarily, nickel is incorporated into stainless steels to enhance corrosion resistance, durability, and strength. To illustrate, austenitic stainless steels containing 8-10% nickel are extensively used in the construction, transportation, and medical equipment industries owing to their outstanding mechanical properties and resistance to oxidation.
Nickel further finds its way into various superalloys that are designed to sustain extreme conditions. Containing as much as 50% nickel, these superalloys are employed in jet engines and gas turbines for aerospace components requiring high-temperature resistance and mechanical stress. According to recent industry data, the global demand for nickel superalloys is expected to rise at a compound annual growth rate (CAGR) of about 6% from 2023 to 2030, thus highlighting their contribution towards advancing aerospace and energy technologies.
Some other nickel alloys prove highly useful in chemical and petrochemical industries as they stand against harsh environments, for example, acidic or high thermal conditions. Monel (nickel-copper) and Hastelloy (nickel-molybdenum) find great use in chemical processing, marine engineering, and power generation.
Nickel alloys are universal enough to be combined for enhanced performance and sustainability improvements in existing industrial sectors. The alloys can be exported as materials with properties of high strength and corrosion resistance. It means that enough design factors could be selected to prevent the waste of materials and curb short lifespan with regard to the present-day green plan. Such abilities make nickel an essential element for industries hoping to settle along with technological innovation while maintaining environmental responsibility.
Use of Nickel in Electronics and Technology
Nickel plays an essential role in electronics and technology due to its unique properties of high conductivity, long wear-time, and corrosion resistance. It has wide applications in batteries, especially lithium-ion and nickel-cadmium batteries, that provide energy to small devices from smart mobile phones to EVs. According to a forecast, global EV demand is expected to witness more than 23% CAGR till 2030, which will create demand for nickel-rich batteries on the flip side.
Nickel further improves semiconductors and electronic circuits, which are the main components of modern devices. Nickel alloys are also used in the production of aerospace components, which need to tolerate harsh temperatures. With progressing technologies, the demand for nickel in 5G infrastructure, IoT devices, and renewable energy systems perpetually increases, affirming its role in creating a sustainable and connected future.
Nickel’s Importance Environmental Applications
Nickel has an important part in environmental sustainability due to its diverse applications in green technologies. One of its most important applications has been in batteries for electric vehicles (EVs), particularly in nickel-metal hydride (NiMH) and lithium-ion batteries. According to recent market data, demand for nickel in EV batteries is projected to grow by more than 60% by 2030, driven by increasing global efforts to reduce carbon emissions. Nickel increases energy density in batteries to allow longer ranges and better performances in electric vehicles.
Nickel then is critical in renewable energy systems, especially concerning the construction of wind turbines and solar panels. This makes components tougher and more resistant to corrosion, enabling these systems to perform well for long periods. For example, stainless steel, containing nickel, is extensively used in wind turbine blades and support structures. Moreover, the recycling rate for nickel is commendably high, where more than 50% of nickel has been recycled from various end-of-life products, thus contributing to the circular economy and lowering environmental impact.
Emerging research efforts are also investigating its application in carbon capture and storage systems as a catalyst for the conversion of carbon dioxide into value-added compounds. This further strengthens nickel’s significance in climate remediation and the support of global decarbonization initiatives. The increasing application of nickel across these proxies thus accentuates its relevance in promoting a cleaner, greener future.
Some Comparative Aspects of Nickel against Other Metals

The most special feature to stress about nickel when compared to other metals would be the inherently unique combination of strength, corrosion resistance, and versatility in alloying. Against most other metals, nickel is used in important renewable energy-related technologies, such as electric vehicle batteries and carbon capture systems. Its unique set of properties hence becomes more important in applications around sustainability and decarbonization when compared to other alternatives.
Density Comparisons: Nickel vs. Other Common Metals
Nickel, iron, copper, aluminum, and titanium are some of the most common metals compared for density.
| Metal | Density | Strength | Corrosion | Versatility |
|---|---|---|---|---|
| Nickel | 8.91 g/cm³ | High | High | Excellent |
| Iron | 7.87 g/cm³ | Moderate | Low | Moderate |
| Copper | 8.96 g/cm³ | Moderate | Moderate | Good |
| Aluminum | 2.70 g/cm³ | Low | Moderate | Excellent |
| Titanium | 4.51 g/cm³ | High | High | Good |
This table highlights the density and key attributes of each metal, helping compare their properties effectively at a glance.
Thermal Conductivity of Nickel Compared to Other Metals
Nickel has lower thermal conductivity compared to copper, higher than iron and titanium, and is similar to aluminum.
| Metal | Thermal Conduct. (W/m·K) | Comparison |
|---|---|---|
| Nickel | 90 | Moderate |
| Copper | 401 | High |
| Iron | 80 | Low |
| Aluminum | 237 | Moderate |
| Titanium | 22 | Low |
This table provides a concise overview of how nickel’s thermal conductivity compares to other commonly used metals, helping in material selection based on thermal performance.
Atomic Radius vs. Density: Understanding
Atomic radius and density are two fundamental properties giving clues about the structural and physical behavior of elements. Considering these properties, the atomic radius is the distance from the nucleus of an atom to its farthest electron, whereas density is mass per unit volume. However, these properties are interrelated, as the atomic arrangement and spacing ultimately define the density of the material.
Atomic Radius and Density of Selected Metals
| Metal | Atomic Radius (pm) | Density (g/cm³) | Relationship |
|---|---|---|---|
| Nickel | 124 | 8.90 | Compact with moderate atomic size and high density |
| Copper | 128 | 8.96 | Slightly larger atomic radius with high density |
| Iron | 126 | 7.87 | Moderately compact structure and lower density than nickel or copper |
| Aluminum | 143 | 2.70 | Larger atomic radius leading to significantly lower density |
| Titanium | 147 | 4.51 | Larger radius, resulting in lower density despite structural strength |
The table above shows that metals with smaller atomic radii tend to have higher densities on account of closely packed atoms. The scenario is inversed with aluminum and titanium having higher atomic radii, thereby lowering density. This relationship depends on both atomic mass and the packing pattern of the material.
Understanding the association between atomic radius and density assumes a major role in applications wherein properties of weight and materials are a matter of design and performance, such as aerospace and electronics.
Latest Advancements and Discoveries in Nickel
I am thinking that some of the latest developments and innovations in nickel involve the advancements of nickel alloys for more efficient applications in high-temperature and corrosive environments, especially in aerospace and energy sectors. Additionally, advances in nickel recycling technologies have made the world more sustainable, supporting the global agenda to conserve resources.
Innovations in the Extraction and Processing of Nickel
Extraction and processing of nickel have witnessed a number of advancements that have upgraded their efficiency, sustainability, and environmental friendliness. One of these key advances is the hydrometallurgical technique. High Pressure Acid Leaching or HPAL is the more common one among these. HPAL permits the efficient extraction of nickel from laterite ores, which are abundantly available but difficult to process using conventional methods. Industry reports have already highlighted the increased number of HPAL-based projects, with some operating at more than 90% recoveries.
Another major recent innovation is the adoption of carbon capture, utilization, and storage (CCUS) amidst nickel processing plants. While capturing carbon emission during extraction, nickel producers are syncing themselves to the global net-zero target. For example, some leading mining companies reported having cutting greenhouse gas emission by up to 30% through the application of CCUS mechanisms.
From the recycling perspective, the enhancement of pyrometallurgical techniques made it possible to recover nickel efficiently from old batteries and stainless steel. According to the International Nickel Study Group (INSG), now about 35% of nickel supply is sourced from recycled nickel, thus emphasizing circular economy practices.
These innovations in extraction, processing, and recycling are what supply the increasing demand for nickel, which is basically driven by its indispensability for electric vehicle (EV) batteries. Electric vehicle (EV) industries are constantly on the rise, and it is expected that by 2030, the demand for nickel will grow by 20% every year, thus putting a lot of importance on sustainability and efficiency of processes to secure its existence in the long term.
Research on Nickel Alloys and Their Future Applications
Over and beyond applications, Is Nickel Alloys are critical materials well known for their strength, corrosion resistance, and resistance to extreme temperature. Therefore, they find application in a large high-service environment, including aerospace, power generation, and medical technology. Based on recent studies, the market for nickel alloys worldwide is expected to grow at around 4.9% CAGR and will touch $17.2 billion by 2028.
Nickel alloys, especially Inconel and Monel, find major application in aerospace for jet engine and turbine manufacture. These alloys withstand thermal fatigue and have engineering properties that guarantee system reliability and life in aircraft applications. The other important area for nickel alloy applications is the energy sector: nickel alloys are essential in nuclear reactors, heat exchangers, oil, and gas pipelines since they can stand against high temperature and corrosion.
Nickel alloys have accordingly been slated to play a key role in emerging technologies from the future standpoint. For example, an expanding EV market requires cell technologies deploying nickel-rich cathodes to increase energy density and vehicle range. The demand for nickel alloys to construct hydrogen production plants and fuel cell systems is further stimulated by green hydrogen promotion as a clean energy carrier. Recent estimates show that nickel demand in EV batteries alone is forecasted to hit 1.8 million metric tons annually by the year 2030, pointing to a considerable growth trajectory.
Growing applications of additive manufacturing, or 3D printing, create new opportunities for nickel alloys. With advanced 3D printing technologies, even complicated components can be engineered at a fine scale, which allows for making lightweight and tough parts for robotic technologies and satellite development among others.
Sustainability optimization of nickel alloy production is under the spotlight as well. Recycling programs and green extraction methods are perceived as essential for reducing the environmental impact of nickel-based fabrication. Around 60% of the nickel used in alloys is already derived from recycled materials, underlying efforts by industry to go sustainable.
However, nickel alloys will continue to advance in technology for many fields. Within durability, versatility, and sustainability, nickel alloys will be among the key materials for 21st-century challenges and innovations.
Impact of Nickel Research on Sustainability
Nickel research is vital in fostering sustainability through efficient and environmentally proprietary materials. For instance, the nickel industry is largely instrumental in the production of stainless steel that is 100 percent recyclable and predominantly used in construction and infrastructure. Latest reports stated that about 90 percent of stainless steel is recycled after an end life. Such a high recycling rate means less down on extraction of primary nickel, conserving natural resources and mitigating environmental degradation.
Moreover, nickel forms an important component for energy storage systems, like batteries for electric vehicles (EVs) and renewable energy applications. Nickel-based batteries, especially lithium-nickel-manganese-cobalt (NMC) chemistries, have higher energy density and better performance. According to BloombergNEF, due to the global push toward cleaner energy solutions, demand for nickel in EV batteries will grow five times by 2030.
Nickel alloy innovation research also allows industrial processes to become more energy-efficient. For example, nickel alloys are used in high-temperature applications such as gas turbines and power plants to improve their efficiency and reduce greenhouse gas emissions. These align with the goals of various international agreements such as the Paris Agreement, prioritizing greenhouse gas emissions reduction.
Then, the industry’s producers continue to make increased commitments to sustainable methods, including carbon-neutral manufacturing and fair-trade programs, to name a few. For example, major nickel producers are now reducing CO2 emissions considerably by the use of advanced smelting techniques and by switching to renewable energy sources. These ongoing efforts make evident that nickel ensures a sustainable future.
Reference sources
1. The Density of Nickel in the Superheated and Supercooled Liquid States
- Authors: S. Y. Shiraishi, R. G. Ward
- Journal: Canadian Metallurgical Quarterly
- Publication Year: 1964
- Citation: (Shiraishi & Ward, 1964, pp. 117–122)
- Summary: This study utilized a levitation melting technique to measure the density of liquid nickel between 1149 and 1866°C. The findings indicated that the density varies linearly with temperature over the entire range studied, showing no discontinuity at the melting temperature. The density was expressed by the relation: ρ(g/cm3)=9.966−12.000×10−4T(°K)±0.055ρ(g/cm3)=9.966−12.000×10−4T(°K)±0.055
- Methodology: The authors employed levitation melting and Archimedean methods to obtain density measurements, comparing results to ensure accuracy.
2. Density of nickel + nickel chloride liquid mixtures
- Authors: J. Galka, J. Moscinski, L. Suski
- Journal: The Journal of Chemical Thermodynamics
- Publication Year: 1972
- Citation: (Galka et al., 1972, pp. 849–856)
- Summary: This paper discusses the density of nickel and nickel chloride mixtures, although it does not focus on the density of nickel alone. The study provides insights into the thermodynamic properties of these mixtures.
- Methodology: The authors conducted experimental measurements to determine the density of the mixtures at various temperatures.
3. Tuning electron density of metal nickel by support defects in Ni/ZrO2 for selective hydrogenation of fatty acids to alkanes and alcohols
- Authors: J. Ni et al.
- Journal: Applied Catalysis B: Environmental
- Publication Year: 2019
- Citation: (Ni et al., 2019)
- Summary: This study explores how support defects in Ni/ZrO2 can influence the electron density of nickel, which is crucial for its catalytic activity in the selective hydrogenation of fatty acids. The findings suggest that tuning the electron density can enhance the selectivity and activity of nickel catalysts.
- Methodology: The authors utilized density functional theory (DFT) calculations to analyze the effects of support defects on the electron density of nickel and its catalytic performance.
Frequently Asked Questions (FAQs)
What is nickel density?
The density of nickel is about 8.90 g/cm³. The high density that the metal is endowed with is indeed favorable for the industrial usages of the metal, such as electronics and construction.
Does oxidation state affect nickel in any way?
Nickel state of oxidation can vary; the more common are the +2 and +3 states. Such variance allows nickel to form a plethora of compounds, such as nickel oxide and nickel carbonyl, each of which has its own peculiar properties and uses.
What are the physical properties of nickel metal?
Nickel is a lustrous, silvery-white metal known for its ductility and corrosion resistance. It has a high thermal conductivity and is utilized extensively in alloys and plating to develop durability against corrosion.
Where does nickel appear in a Periodic Table?
It is located in group 10 of the Periodic Table of Elements, with an atomic number of 28 and the chemical symbol Ni. It is a transition metal and shows the characteristics typical of the group.
What are the isotopes of nickel?
Nickel has more than several isotopes, with the common being Ni-58, Ni-60, Ni-61, and Ni-62. Differing numbers of neutrons result in varied atomic numbers for different isotopes.
What is the atomic radius of nickel?
The atomic radius of nickel is around 124 pm. Being significant in its description of the atomic pattern and bonding behavior, nickel in various compounds is thus measured.
How is nickel used in industries?
Nickel stands as a crucial element in nearly every industry-from stainless steel to batteryries and electronic components. Due to its corrosion-resistant quality and thermal conduction capacity, it is used for such purposes.
What is the relation between nickel and chromium?
Nickel and chromium are often combined in alloying to produce stainless steel, which is also the most corrosion-resistant metal. The combination enhances the physical and chemical properties, making it suitable for a wider range of applications.
- Sulfuric Acid Solution: Density and Concentration Guide
- Buying a Cheap Laser Cutter: Pros and Cons of Budget Laser Machines
- Density of Steel: Mild and Carbon Steel Density Explained
- Complete Guide to Laser Marking Machines: How Laser Marking Systems Work
- CNC Router Vs Laser Cutter: Which Cutting Machine is Right for You?
- Understanding the Caffeine Melting Point: Approximately 236 Degrees
- Engrave with Precision: The Ultimate Guide to CO2 Laser Marking Machine on Wood
- Mastering Fiber Optic Laser Technology: The Ultimate Guide to Fiber Laser Cutting and Beyond

